What are NSCs?
Stem cells are generally defined as uncommitted cells that can divide repeatedly while maintaining potency to generate differentiated cell types. The term neural stem cells (NSCs) is used loosely to describe cells with the ability to self-renew and to generate different cell types through asymmetric division, that can give rise, primarily, to neuronal (neurons) and glial (astrocytes and oligodendrocytes) lineages1.
In the developing central neural system (CNS) NSCs display very complex biology and undergo a series of transformations from neuroepithelial cells, through radial glia, to adult neural stem cells with astroglial properties, resulting in different progeny at different developmental stages2.
The establishment of protocols for the extraction or differentiation of these cells and their expansion in vitro was a great advancement in neurobiology. NSCs grown in culture provide a powerful tool and an accessible model to investigate human neurodevelopment and cell biology.
They also offer a renewable source of cells suitable for pharmaceutical and toxicological screening studies3. And very importantly, there is a promise that in the future NSCs could be either stimulated to enhance the endogenous CNS repair mechanisms or could provide a cell source for stem cell therapies to replace damaged or dysfunctional cells following an injury or in neurodegenerative diseases such as amyotrophic lateral sclerosis, Parkinson’s disease, Alzheimer’s disease, or Huntington’s disease.
NSC sources and isolation methods
Today, there are three main sources of NSCs in culture.
They can be derived by direct isolation from primary CNS tissue, such as fetal and adult brain and spinal cord tissue. This method is the fastest, easiest and provides NSCs directly from the nervous tissue, which have properties that are well characterized. However, the limited amount of available tissue and obvious associated ethical issues are the major drawbacks, especially for NSC-based cell therapies.
NSCs can be obtained also by the differentiation of pluripotent stem cells such as embryonic stem cells (ESC)5 and more recently induced pluripotent stem cells (iPSCs)6. There are various protocols to achieve this, but the majority share common progress of inducing the formation of embryoid bodies, followed by the formation of morphologically very distinct structures known as neural rosettes. The latter are thought to represent the early neural tube, that is present in development and harbor the NSCs7. These can be then isolated, expanded as monolayers, and differentiated into mature neural cells.
iPSCs derived NSCs circumvent ethical issues associated with embryonic/fetal cells. Moreover, iPSCs derived from patients with neurological diseases hold the promise for modeling human diseases and deepening our understanding of the underlying mechanisms. Their main downside is the labor-intensive, lengthy, and costly process of deriving, maintaining, and differentiating these cells8 as well as aberrations introduced by the reprogramming process.
And finally, several protocols were developed that derive NSCs by transdifferentiation of somatic cell types, primarily fibroblasts9 but also mesenchymal stem cells, liver or urine cells. In this case, the cells are directly reprogrammed into neuronal stem cells, bypassing the pluripotent stage, therefore shortening the process. These cells however remain to be thoroughly characterized.
Isolation of human NSCs from fetal brain tissue
There are several detailed protocols10 on how to isolate NSCs, however, the main principle is always the same with only minor modifications:
- Desired CNS region (whole brain, cortex, hindbrain, spinal cord, etc) is microdissected from 6-10 weeks old human embryos.
- Tissue is then enzymatically or mechanically dissociated to a single cell suspension.
- Cells are plated in a serum-free medium supplemented with growth factors stimulating the proliferation of the stem cells.
- To get a homogenous culture of NSCs and eliminate any committed progenitors or differentiated neuronal cells, the culture then goes through a neurosphere stage. The cells are replated on a surface that does not support neural cell attachment (e.g. gelatin coating). NSCs will form small clusters and continue to proliferate, while differentiated cells will die.
- The formed neurospheres will continue to grow in suspension or can be dissociated and replated on extracellular matrix substrate (e.g. laminin)-coated dishes and expanded as monolayers.
During this isolation process, the morphology of the cultures changes rapidly through the different stages; therefore, observing the cells on regular basis is important to check the progress and ensure successful isolation of NSCs.

Neurospheres vs. adherent cultures
Neurospheres represent the original method for isolation and expansion of NSCs11. They are non-adherent aggregates of NSCs that need to be continuously passaged, approximately every 5-7 days, before they become too large (more than 200 mm in diameter) to prevent the formation of a necrotic core as well as spontaneous differentiation into more committed neural progenitors and differentiating cells10.
Multiple studies have shown that neurospheres present high heterogeneity of cells and can progressively lose their self-renewal and differentiation. Neurospheres have a tendency to acquire different fates depending on their size and frequency of passaging12,13. Therefore, monolayer cultures of NSCs seem to be the more favorable method.
Adherent monolayer cultures of NSCs were developed as an alternative to neurospheres and they offer several advantages, including increased expansion potential, more stable phenotype over time in culture, and lower risk of spontaneous differentiation14. Cells in monolayer cultures can be quickly and easily observed using brightfield microscopy. This allows for any changes in morphology, which could be indicative of phenotype changes, to be monitored.
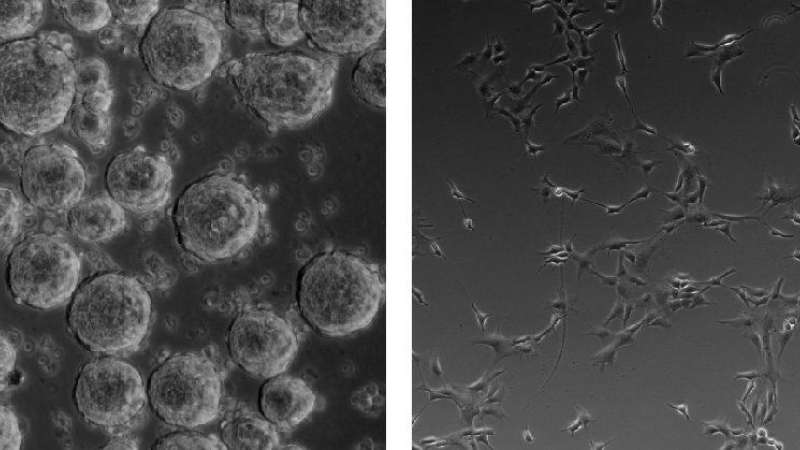
Neural stem cell culture conditions
NSCs are generally cultured in serum-free medium to keep the cells in the undifferentiated state and supplemented with neural cell supplements, B27 and N2, as well as growth factors promoting their proliferation, such as epidermal growth factor (EGF) and fibroblast growth factor FGF-210,15.
Appropriate extracellular matrix (ECM) coating allows the cells to attach with laminin and laminin-rich matrices, with Matrigel being the most favorable. While rodent NSCs can also grow on collagen substrates, human NSCs show a very strong preference for laminin, reflecting the composition of the ECM in NSCs niche in vivo16,17.
Like all stem cells, NSCs are very sensitive to changes in their environment that can affect their properties; therefore, morphology, confluency, and viability need to be monitored regularly to ensure good quality cultures. Rounding up of cells, excessive cell debris, and floating cells indicate issues with viability of the culture. Clustering and clumping of cells, pinching off of neurospheres indicate insufficient or unfavorable ECM coating.
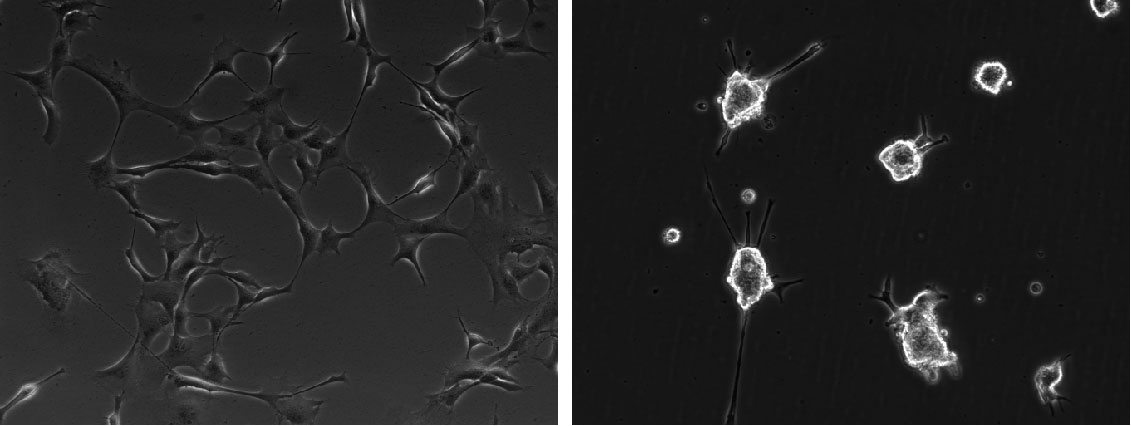
Adherent NSC cultures proliferate steadily and double every 2-3 days. The cells should never be allowed to reach confluency as it can induce changes in their properties and trigger spontaneous differentiation. Ideally, they should be split at 80-90% confluency, at a ratio between 1:3 to 1:6. Splitting the cells too sparsely, can also negatively affect the quality of the cultures, which can be characterized by slowing down of proliferation and/or a more flattened appearance.
-----------------------
For researchers looking to continuously monitor the health of their cells remotely and in real-time, we recommend looking at the CytoSMART Lux2 and the CytoSMART Lux2 Duo Kit. These brightfield microscopy systems with a cloud connection are designed to operate from within cell culture incubators. No need to enter the lab for routine inspection rounds, you can simply observe your cells from your phone or PC, via your personal account.
-----------------------
Neural stem cell properties
Human NSCs are relatively small and present two types of morphologies: small bipolar cells or flatter apolar cells. These are not separate populations as the cells are readily able to change between these morphologies. Real-time monitoring of NSCs also reveals that they are very motile.
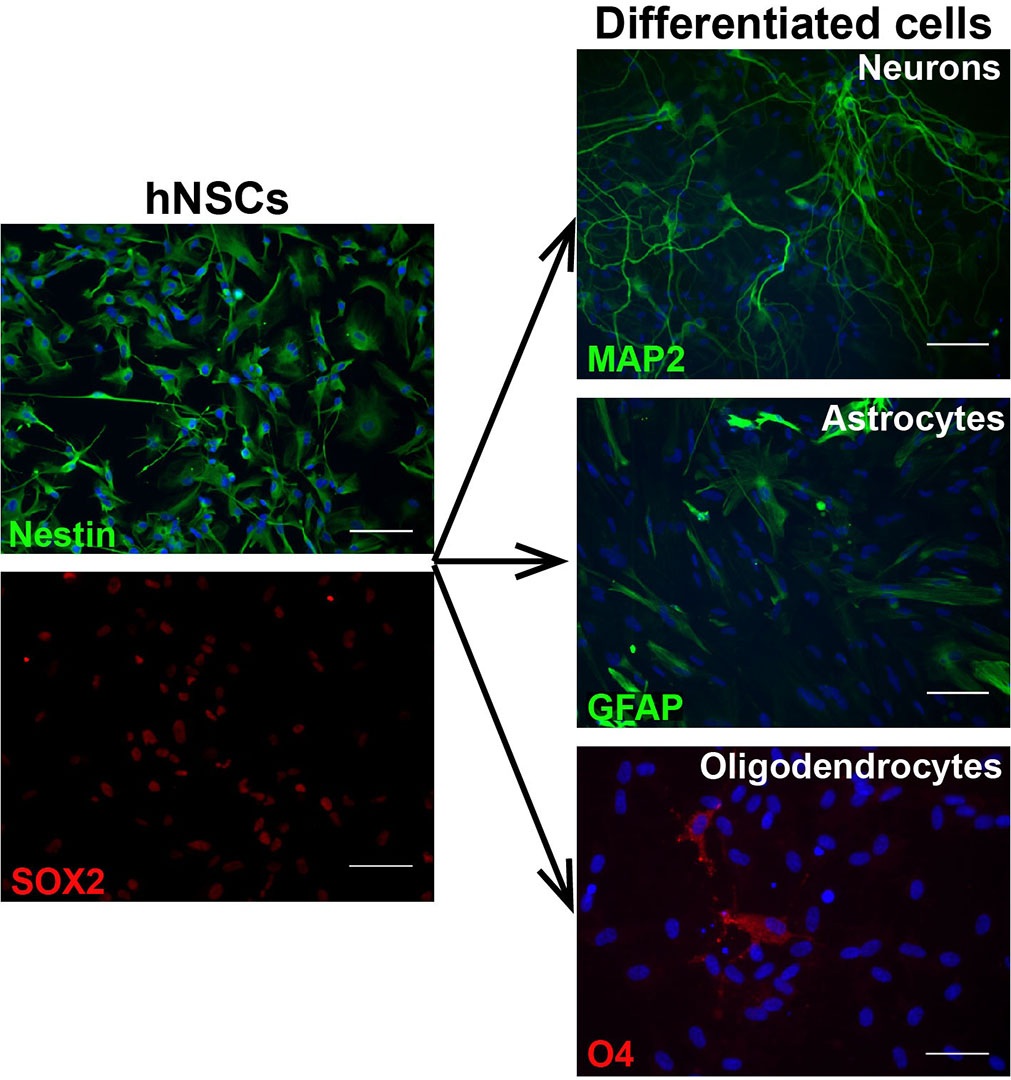
NSCs homogenously express markers such as nestin and SOX2. Human NSCs in culture show similarities with radial glia, both in morphology and expression of molecular markers, including FABP7 (BLBP), GLAST, Pax6, and GFAP10,16.
Differentiation of neural stem cells
Another important characteristic of NSCs is their tripotent differentiation potential, or ability to generate astrocytes, oligodendrocytes, and mature neurons indefinitely, even after prolonged expansion3,10.
NSCs grown in culture medium containing serum, differentiate towards astrocytes, as gliogenesis is their “default” differentiation program. Previous studies confirmed that bone morphogenetic protein 4 (BMP-4) is the main active component in the serum driving the differentiation and it can be used to induce astrocyte differentiation also in serum-free conditions12,18. NSCs change their appearance rapidly upon differentiation and become larger and flatter with larger nuclei.
Neuronal differentiation of NSCs in most protocols is triggered by gradual removal of the growth factors (EGF and FGF) from the expansion medium, which slows down cell proliferation and induces changes in their morphology3,10. NSCs become smaller and more spindle-like with thin processes. Some clustering and cell death can be also observed in this initial phase. Maturation of the neuronal cells is supported by supplementing the maintenance medium with growth factors such as brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF).
Lastly, NSCs can be differentiated into oligodendrocyte precursors although the efficiency is very low, especially in human cells, with only about 1-2% O4 positive cells (with O4 being the most common marker for these cells) after 5 weeks in culture10. Molecules and factors that are able to promote survival and maturation of rodent oligodendrocyte precursor cells from NSCs do not seem to be similarly effective in human cells, therefore there is an urge to develop new protocols and strategies14 .
Cell differentiation needs to be confirmed by examining the expression of appropriate markers, however, NSCs differentiating towards the individual lineages show substantial morphological changes that can be easily monitored throughout the process.
Concluding remarks
NSCs are not only a promising source for cell therapies but also a great model to study neurodegenerative diseases. Using them to establish robust in vitro systems, which mimic in vivo mechanisms, is an invaluable tool that paves the way to understand biological systems in health and disease. These culture systems are relatively inexpensive and because their morphology is so prone to change, the live cultures are easy to monitor. However, despite numerous NSCs culture and differentiation systems available, which are reproducible and controllable, there is a need to further improve the established protocols to maximize the potential that these cells offer to researchers.
Related Products
There are currently no products tagged to this resource.