What are MSCs?
Mesenchymal stem or stromal cells (MSCs) were first identified in 1966 to describe a small subpopulation of bone marrow cells that demonstrated osteogenic potential1. These cells also had a unique set of physical characteristics that included a rapid adherence to tissue culture vessels and a spindle-like appearance of their progeny in culture.
Over the years, the term has come to be used more generally to describe any undeveloped biological cell population that is capable of proliferation, self-renewal, and regenerating tissues2.
Today, MSCs encompass a range of cell types derived from diverse tissues with varying levels of multipotency3. The term has also come to be used synonymously with a plethora of other names, such as marrow stromal cells, multipotential stromal cells, mesodermal stem cells, mesenchymal progenitor cells, and medicinal signaling cells4–6. This has inevitably resulted in confusion and misunderstanding giving opportunity for exploitation by companies that claim to market stem cell-based treatments.
Researchers in the field have in fact argued to stop using the term altogether3. In light of this, the International Society for Cellular Therapy has put forward a list of minimal criteria to universally define MSCs. This includes the ability to adhere to plastic, expression of a set of surface antigens, and multipotent differential potential under standard in vitro differentiation conditions7.
In summary, MSCs refer to tissue-specific stem progenitor cells3.
Sources of Human MSCs
Throughout the vasculature of the human body, there exists a tight network of perivascular niche. This is the origin of MSCs in the body and so in principle, MSCs can be derived from all human tissues8,9. However, so far these have been successfully isolated from a handful of specific tissues that include bone marrow, adipose tissue, umbilical cord, dental tissue, skin, liver, bile ducts, salivary gland, tendon, lung, and the periosteum10.
MSCs have different properties depending on source tissue
MSCs isolated from different tissue sources in the body were initially reported to share similar characteristics in vitro, such as plastic adherence, proliferation capacity, immunophenotype, and multilineage differentiation ability9.
However, an accumulation of data has now shown that there is widespread heterogeneity in the proliferation and differentiation potential of MSCs depending on the tissue source. As a consequence, research has focused on predetermining the most appropriate MSC source for a range of applications11,12.
Currently, three MSC sources have come to dominate research and clinical studies for autologous cell-based therapies due to their ease of harvest, potential capabilities, and accumulation of research data. Among these, the most widely used source of MSCs in clinical trials is the bone marrow, followed by adipose tissue and umbilical cord. These three sources account for the vast majority of clinical trials among the 1,043 MSC-related trials that were conducted between 2011-2018 with around 47,000 patients4,13.
Isolation of MSCs
Unlike other stem cells, such as the hematopoietic stem cells, which can be easily identified and isolated by the expression of a specific marker such as the CD34 surface marker, MSCs do not express a distinct marker. This makes the isolation and characterization of MSCs challenging. However different markers have come to be used for this purpose depending on the source of isolation. For instance, the CD105 surface antigen has been used in recent times to isolate human MSCs from the bone marrow11.
There is currently a lack of standardized protocols for the isolation and cultivation of MSCs from different sources. This is an important reason for obtaining MSCs with varying properties.
For instance, let us consider how bone marrow-derived mesenchymal stem cells are isolated. First, mononuclear cells are separated from bone marrow aspirates. Second, these are transferred into cell culture plates or flasks coated with gelatin. Third, within few hours MSCs adhere to tissue culture plastic, and the rest of the cell population including red blood cells or hematopoietic progenitors can be discarded. Lastly, flow cytometry is used to allow sorting of bone marrow-derived MSCs for specific surface markers.
Additionally, a common assay for MSCs can be performed called the colony-forming unit fibroblast assay (CFU-F). In this, cells are plated at low density in large plates so that each MSC generates a colony. In this way, one can measure approximately how many MSCs are present in a given population of cells14.
Culturing of MSCs
Optimum culture condition is a key factor for the efficient proliferation and differentiation of MSCs. This includes an optimum quality of the fetal calf serum, basal medium, glucose concentration, stable glutamine, bone marrow mononuclear cell plating density, MSC passaging density, and the plastic surface quality10. Extensive research has been conducted to find optimal choice for each of these factors and media constituents in MSCs culturing. Several companies also sell culture and expansion media for MSCs.
For instance, obtaining and maintaining optimum confluency of cells is important, since lower and higher cell confluency is known to affect cell viability, as well as growth and differentiation potential of MSCs15–17.
Rapidly self-renewing MSCs are morphologically characterized as elongated, fibroblastic-like, spindle-shaped cells with MSC surface markers. Whereas, large cuboidal or flattened cells have been shown to be slow in replication and proliferation18.
There is an urgent need to combat the heterogeneity that arises in MSC characteristics due to the differing conditions of MSC production, culturing, and monitoring. Here, automating these labor-intensive processes might provide a key solution towards combating product variability and addressing risks of contamination, leading to robustness, reproducibility, and efficacy that is required for the therapeutic application of MSCs19,20.
-----------------
For researchers looking to do live-cell imaging for culture quality control, we recommend looking at the CytoSMART Lux2 Duo Kit. Automated imaging from within cell culture incubators to provide real-time insight into the current state of the confluency of the culture.
------------------
Differentiation of MSCs
Differentiation of MSCs into a desired cell type is dependent on the three factors mentioned before. This includes optimum initial seeding density, medium conditions, and the addition of additives such as proteins or growth hormones to the culture media21.
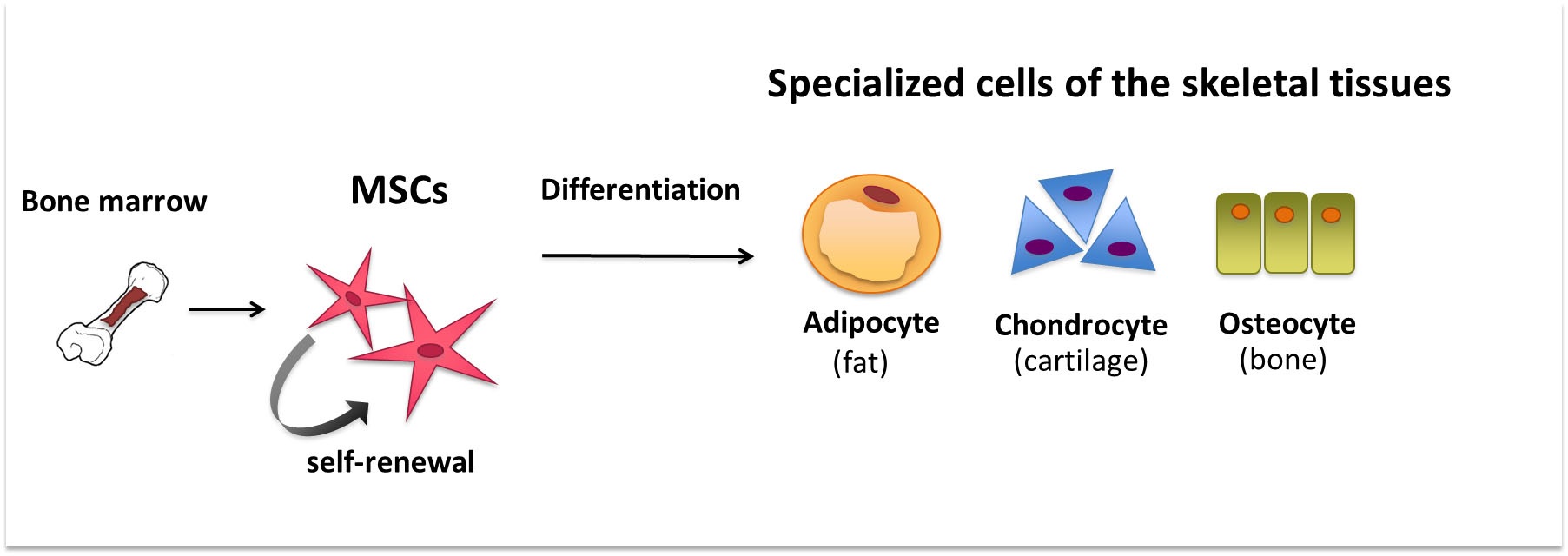
Trilineage differentiation towards osteogenesis, adipogenesis, and chondrogenesis is common for bone marrow-derived MSCs, and several companies sell induction media to facilitate this. Upon differentiation, the expression level of marker genes can subsequently be evaluated by quantitative RT-PCR.
Epigenetic regulation has also been recently found to influence MSC differentiation and proliferation, while more research into this is currently ongoing22.
MSCs can differentiate into a variety of cells of mesodermal lineage, such as adipocytes, osteoblasts, myocytes, chondrocytes, endothelial, and vascular smooth muscle cells (see Figure 1). Differentiation into non-mesenchymal cells such as hepatocytes, neurons, and pancreatic islet cells has also been observed in vitro when specific culture conditions and stimuli are applied23.
Therapeutic Potential of MSCs
MSCs possess several properties that are advantageous in the field of regenerative medicine and tissue engineering. Their potential for immunomodulatory, immunosuppressive, and regenerative properties has been exploited in the form of cell-based therapy for inflammatory, immune-related, and degenerative diseases25.
MSCs have the ability for selective migration toward a site of injury, in a process called homing. This means they can act as an efficient surveillance system, which detects changes in the environment such as the presence of inflammation. They have in fact been shown to travel to the site of inflammation from the injection site and subsequently induce the release of bioactive reagents26.
Their use is beneficial in tissue regeneration given their capability to differentiate into several cell lineages, their homing capacity, angiogenesis, anti-apoptotic activity, and secretion of bioactive soluble factors.
MSCs function in immunomodulation by inhibiting the proliferation of several types of immune cells through the action of cytokines and regulatory factors. Their anti-inflammatory effect decreases an immune response to inflammation, thus protecting the host. Anti-apoptotic activity of MSCs protects injured cells and preserves organ function25.
Given these potential capabilities of MSCs, their use is currently being investigated for cardiovascular diseases, spinal cord injury, bone and cartilage repair, and autoimmune diseases. Let us look at some of these therapeutic areas more closely.
Neurodegenerative disorders
MSC therapy is considered to be amongst a few promising treatment options for neurodegenerative disorders, such as multiple sclerosis, amyotrophic lateral sclerosis, Parkinson's disease, Huntington's disease, spinal cord injury, and Alzheimer's disease. This is based on early beneficial effects that were reportedly observed in a few pilot clinical studies27.
However, for many of these disorders, the responsible pathophysiological events are still not fully understood, and researchers have used MSCs as a working hypothesis27.
It is unclear if the benefits of MSC therapy in neurodegenerative disorders are rendered through the differentiation of MSCs into functional neural cells or merely as a result of their stromal action through the secretion of neural growth factors. Here the precise mechanism of how neural tissue repair occurs upon introduction of MSCs is still unclear.
Bone defects
Site‐directed delivery of MSCs that can be locally implanted in association with biomaterials is being tested to induce skeletal regeneration for bone defects. Some beneficial effects of MSCs have been observed in the treatment of meniscus, intervertebral disc, ligament injuries, osteoarthritis, osteonecrosis, nonunion bone defects, infantile hypophosphatasia, osteoporosis, bone fractures, and osteogenesis imperfecta27.
Cardiovascular disorders
Cardiovascular disorders including acute myocardial infarction and ischemic cardiomyopathy that result from an excessive immune response of the body, can potentially be treated through the anti‐inflammatory potential of MSCs. This is one of the areas that is currently under investigation for MSC therapy. Promising results were obtained with infarcted murine hearts, where injected MSCs became functionally integrated with the surrounding native myocardium. This finding contributed towards increasing expectation for MSCs to be able to differentiate into cardiomyocytes27.
Impediments to clinical use
It has been over 50 years since the first identification of MSCs. The ensuing years have revealed their enormous potential, along with their ease of isolation and assurance of safety. However, this has not been translated to the clinics.
In 2018, only nine products related to MSCs had made to market authorization worldwide. Although there were about 900 clinical trials related to MSCs in the early 2019, only 50 of them were in phase III. There are several factors as to why the therapeutic application of MSCs has been lacking. This includes amongst others, a need for better understanding of MSC sources, delivery manner, and cell doses27.
MSC-based pharmacological developments are currently missing robust pharmacodynamic and pharmacokinetic models of these cells and their bioactive reagents. These involve robust investigation into the recipient biological environment, as well as the characterization of the size, source, culture conditions, and route of administration of MSCs28. Addressing these limitations that are currently being faced will facilitate the use of MSCs in several different clinical applications.
Many of the target diseases for MSC therapy are systemic in nature. This is a problem such as when treating systemic tissue defects with MSCs. The preferred method in treating tissue defects is for local implantation directly into the injured site. Since for systemic disabilities, this is not possible, an intravenous administration of MSCs is required. This puts into question the biodistribution of these cells when introduced into the body27.
Research has shown that the first organ these cells reach are the lungs and most of the infused MSCs get trapped there. This is followed by the phagocytosis of a few MSCs by monocytes which in turn migrate to other specialized body sites. This can be exploited to promote immunosuppression as seen when infused apoptotic MSCs acted together with monocytes in an animal model of graft versus host disease27.
However, low cell survival rate and differentiation capacity in vivo after MSC transplantation have been observed and this puts into question the effectiveness of such MSC therapy12.
Conclusion
MSCs have a long and intensive history of research due to their promising range of applications. However, in spite of this, clinical benefits are still lacking. There are many reasons why it has been difficult to translate the potential of MSC therapy into therapeutics. Standardization and automation of research protocols for the different parameters of MSCs will provide a key step in this direction. There is a need for better understanding of the underlying mechanisms that regulate and modulate MSCs.
Although there is a lot that remains to be learnt about the biology and applicability of MSCs, it should be appreciated that MSC therapy holds great promise for many untreatable disabilities of the present time.
Related Products
There are currently no products tagged to this resource.