Introduction
Cell outgrowth assays, also referred to as nest assays or radial migration assays, can be used to investigate collective cell migration in fields such as oncology and drug discovery1–5. The outgrowth assay is essentially the opposite of the cell exclusion zone assay and wound healing assay in that cells expand outward from a nest as opposed to inward to close a void6,7. A further difference between the outgrowth assay and the wound healing assay is that it reflects the normal cell microenvironment better8. Both cell outgrowth assays and cell exclusion zone assays resemble the normal cell microenvironment; therefore, these two assays share many of the same advantages. The two main benefits of the outgrowth assay are that the cell monolayer is not wounded and the cell-free surface can be defined before migration9,10.
There are a few disadvantages to the outgrowth assay that should be taken into consideration when designing an experiment. Firstly, the initial amount of cells in the nest cell number may have an effect on the rate of migration. Therefore it is important to repeat assays with different initial cell densities so that these effects can be observed and quantified11. In addition, the geometry of the initial cell area affects cell migration and, therefore, the outcome of the assay9. For most experiments, however, the geometries used in outgrowth assays are either square or circular7. Well-designed experiments can mitigate these disadvantages. The following sections will elaborate on the various outgrowth assays available to researchers. An overview is summarized in Figure 1.
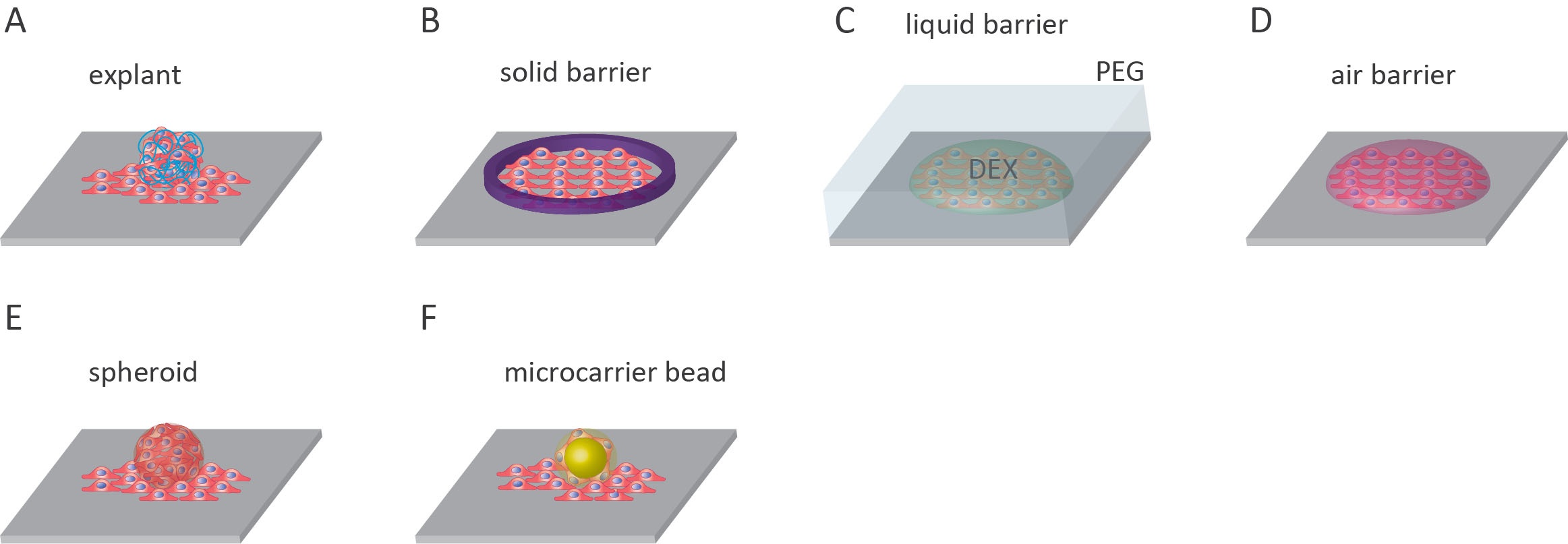
Explant outgrowth assays
One assay that lies between in vivo studies and in vitro experiments is the explant outgrowth assay. The method involves the collection of tissue samples from an organism, culturing tissue specimens, and monitoring the outward migration of cells from these explants12. This assay has been used to monitor the outgrowth of keratinocytes from skin explants, study glomerular diseases using outgrowth from kidney explants, investigate tenocyte migration from tendon explants, and examine lymphangiogenesis using lymphatic duct explants12–15. The advantage of using explants in cell outgrowth assays is that the unique features present within the diseased tissue are preserved12.
Solid barrier
The most common outgrowth assay features a solid barrier to initially confine cultured cells before migration. This migration assay is most commonly known as the fence assay16. Cells are limited to an area of the microplate with the use of a solid barrier. The barrier is usually cylindrical, with the cells added inside the cylinder and allowed to adhere to the surface of the microplate. The cylinder can be made from glass or metal-silicone8,16–19. Alternatively, stencils made from poly-dimethylsiloxane (PDMS) can be used to create uniform cell islands on the surface20,21. Before the experiment, the barrier is removed, and any unattached cells are rinsed off. After that, the remaining attached cells migrate onto the surrounding cell-free substrate8,16–19.
The advantages of solid barriers include that they are reusable and that they can be adapted for high-throughput, automated imaging systems3,20. An example of a high-throughput system is the injection-molded gaskets developed by Oliver et al. (2020), which enable the performance of 24 radial migration assays simultaneously. However, drawbacks of this system are the non-uniformity of clamping pressure of the gaskets, the formation of bubbles, and thorough cleaning that is required after each experiment3. To date, only commercial cylindrical barriers are available such as the metal-silicone barriers from Aix Scientifics and Pyrex® Cloning Cylinders available from Fisher Scientific and Sigma8,11,18.
Liquid barrier
The use of aqueous two-phase systems (ATPS) can be applied to both outgrowth and cell exclusion zone assays. In this technique, an ATPS can be produced when solutions of two incompatible polymers are mixed at threshold concentrations. The most well-understood ATPS is the polyethylene glycol (PEG)/dextran (DEX) system. PEG and DEX phase separation occurs at low polymer concentrations and under non-denaturing conditions making it viable for mammalian cells22. In addition to ATPS, the use of other immiscible liquids has been applied to cell exclusion zone assays, though this has not been widely used23.
To set up an outgrowth assay using ATPS, droplets of DEX containing mammalian cells can be printed onto a substrate and covered with a solution of PEG. The cells included in the DEX phase are excluded from entering the PEG phase due to PEG/DEX interfacial tension. A cell-free surface is maintained outside the DEX droplets that can then be used for the assay22,24.
The advantages of ATPS are that these systems are inexpensive to establish and do not require sophisticated equipment. The assay is rapid, compatible with a variety of cell types, and can be automated for high throughput22,24,25. The geometry of the cell-free surface can also be controlled in more sophisticated setups26. The drawbacks of this approach are that the viscosity of the DEX phase can result in increased variability of pipetted volumes, and the DEX droplets can be disrupted when the PEG solution is added22.
Air barrier
The liquid-gas interphase can also be used to generate cell-free surfaces for cell outgrowth assays. In this method, droplets of cells are added to a dry surface and are allowed to adhere (between 30-60 minutes). Following adherence, the surface is then entirely covered in cell medium, enabling the cells to migrate out from original droplets27.
This technique is simple to set up and only requires standard cell culture materials. Limitations of this system include the requirement that cells must adhere rapidly, the cell-free surface is initially dry, and due to the necessity of a short adherence time to maintain cell viability in the droplets, the cell patterning can vary27.
Spheroids outgrowth
Another regularly used assay is the flat surface spheroid migration assay or simply the spheroid migration assay28–31. This assay is a combination of three-dimensional (3D) cell spheroids and the two-dimensional outgrowth assay. In this assay, spheroids, produced from cells in suspension culture, are attached to the microplate surface and measured for outgrowth2,4,5,32. The surface of the plate can be coated to alter attachment affinity. Laminin, which is a strong adhesion protein for epithelial cells, can be used to increase attachment, whereas, agarose can be used to prevent attachment to the surface2,33.
A significant advantage of using spheroids is that, because of their 3D structure, they more closely represent the tissue physiology being studied, such as small cancer clusters7. Another advantage of starting with spheroids is that they can be produced with a consistent diameter. This uniformity makes the assay accurate and easily miniaturized and automated for high-content imaging2. A limiting factor to this assay is that not all cells can form spheroids, and experience with spheroid formation is required7. Commercial plates such as the 15-well µ-Slide Angiogenesis plates by ibidi have been used to prepare gels for spheroid attachment.
Micro-carrier beads
The use of micro-carrier beads is a wholly different approach to tackle reproducibility. Pre-seeded micro-carrier beads provide a highly reproducible basis from which cells can migrate outwards. This assay can be used to study cell migration via analysis of spreading from the point of contact between the bead and culturing surface, or invasion when the bead is embedded in a layer of gel such as fibrin gels34. Beads can be made from hydrated collagen-coated dextran beads35. Commercial beads are available such as Dextran-coated Cytodex 3 microcarrier beads from Amersham Biosciences36. This method yields highly reproducible results; however, there are a few drawbacks. The disadvantages include the cost of the beads, and some beads will be insufficiently coated in cells and must be removed7.
Conclusion
A multitude of outgrowth assays is available to researchers to investigate collective cell migration. These can range in sophistication from spheroid outgrowth assays and ATPS systems to air and solid barriers to create cell nests for expansion. All these assays, except explants, spare the cell monolayer from wounding. The scientist can also specify the substrate onto which cells migrate. The outgrowth can be used in parallel with other cell migration assays. Information relating to collective cell migration techniques, specifically cell removal and exclusion, and single-cell migration assays form part of this series of articles covering methods used in cell migration.
|
Method |
Advantages |
Disadvantages |
Commercial products |
References |
|---|---|---|---|---|
|
Explants |
Preserves features of diseased tissue |
Requires ethics approval, wounding |
- |
12 |
|
Solid barrier |
Reusable, automatable |
Incomplete contact with the surface |
Metal-silicone barriers (Aix Scientifics) Pyrex® Cloning Cylinders (Fisher Scientific and Sigma) |
16 |
|
Liquid barrier |
Basic setup, automatable |
DEX viscosity increase variability |
- |
22 |
|
Air barrier |
Basic setup |
Fast adhering cells required |
- |
27 |
|
Spheroids outgrowth |
Consistent spheroid size, closer to in vivo physiology, automatable |
Limited to a cell that forms spheroids, experience required |
µ-Slide Angiogenesis plates (ibidi) |
2 |
|
Microcarrier beads |
Reproducible |
Expensive |
- |
36 |
References
[1.] Gilmore, T. D., Cormier, C., Jean-Jacques, J. & Gapuzan, M.-E. Malignant transformation of primary chicken spleen cells by human transcription factor c-Rel. Oncogene 20, 7098–7103 (2001).
[2.] Christoffersson, J. et al. A cardiac cell outgrowth assay for evaluating drug compounds using a cardiac spheroid-on-a-chip device. Bioengineering 5, 36 (2018).
[3.] Oliver, C. R. et al. Development of a high-throughput radial migration device. bioRxiv (2020).
[4.] Silva, J. et al. Fibrin functionalization with synthetic adhesive ligands interacting with α6β1 integrin receptor enhance neurite outgrowth of embryonic stem cell-derived neural stem/progenitors. Acta Biomaterialia 59, 243–256 (2017).
[5.] Walker, J., Martin, C. & Callaghan, R. Inhibition of P-glycoprotein function by XR9576 in a solid tumour model can restore anticancer drug efficacy. European journal of cancer 40, 594–605 (2004).
[6.] Madhyastha, H., Radha, K., Nakajima, Y., Omura, S. & Maruyama, M. uPA dependent and independent mechanisms of wound healing by C-phycocyanin. Journal of cellular and molecular medicine 12, 2691–2703 (2008).
[7.] Pettit, D., Horbett, T., Hoffman, A. & Chan, K. Quantitation of rabbit corneal epithelial cell outgrowth on polymeric substrates in vitro. Investigative ophthalmology & visual science 31, 2269–2277 (1990).
[8.] Kramer, N. et al. In vitro cell migration and invasion assays. Mutation Research/Reviews in Mutation Research 752, 10–24 (2013).
[9.] Treloar, K. K., Simpson, M. J., McElwain, D. S. & Baker, R. E. Are in vitro estimates of cell diffusivity and cell proliferation rate sensitive to assay geometry? Journal of Theoretical Biology 356, 71–84 (2014).
[10.] Shiode, Y. et al. A novel cell exclusion zone assay with a barrier made from room temperature vulcanizing silicone rubber. Plos one 12, e0190198 (2017).
[11.] Simpson, M. J. et al. Quantifying the roles of cell motility and cell proliferation in a circular barrier assay. Journal of the Royal Society Interface 10, 20130007 (2013).
[12.] Zhang, J. et al. Microtubule-associated protein 4 phosphorylation regulates epidermal keratinocyte migration and proliferation. International journal of biological sciences 15, 1962 (2019).
[13.] Rötzer, V. et al. Desmoglein 3-dependent signaling regulates keratinocyte migration and wound healing. Journal of Investigative Dermatology 136, 301–310 (2016).
[14.] Maeng, Y., Aguilar, B., Choi, S. & Kim, E. Inhibition of TGFBIp expression reduces lymphangiogenesis and tumor metastasis. Oncogene 35, 196–205 (2016).
[15.] Jackson, J. E., Kopecki, Z., Anderson, P. J. & Cowin, A. J. In vitro analysis of the effect of Flightless I on murine tenocyte cellular functions. Journal of Orthopaedic Surgery and Research 15, 1–14 (2020).
[16.] Luca, A. C. et al. Impact of the 3D microenvironment on phenotype, gene expression, and EGFR inhibition of colorectal cancer cell lines. PloS one 8, e59689 (2013).
[17.] Wang, Z., Sosne, G. & Kurpakus-Wheater, M. Plasminogen activator inhibitor-1 (PAI-1) stimulates human corneal epithelial cell adhesion and migration in vitro. Experimental eye research 80, 1–8 (2005).
[18.] Ammann, K. R. et al. Collective cell migration of smooth muscle and endothelial cells: impact of injury versus non-injury stimuli. Journal of biological engineering 9, 19 (2015).
[19.] Ammann, K. R. et al. Collective cell migration of smooth muscle and endothelial cells: impact of injury versus non-injury stimuli. Journal of biological engineering 9, 19 (2015).
[20.] Folch, A., Jo, B.-H., Hurtado, O., Beebe, D. J. & Toner, M. Microfabricated elastomeric stencils for micropatterning cell cultures. Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 52, 346–353 (2000).
[21.] Park, J. et al. Microfabrication-based modulation of embryonic stem cell differentiation. Lab on a Chip 7, 1018–1028 (2007).
[22.] Frampton, J. P., White, J. B., Abraham, A. T. & Takayama, S. Cell co-culture patterning using aqueous two-phase systems. JoVE (Journal of Visualized Experiments) e50304 (2013).
[23.] Cai, G., Lian, J., Shapiro, S. S. & Beacham, D. A. Evaluation of endothelial cell migration with a novel in vitro assay system. Methods in cell science 22, 107–114 (2000).
[24.] Tavana, H. et al. Rehydration of polymeric, aqueous, biphasic system facilitates high throughput cell exclusion patterning for cell migration studies. Advanced functional materials 21, 2920–2926 (2011).
[25.] Lemmo, S., Nasrollahi, S. & Tavana, H. Aqueous biphasic cancer cell migration assay enables robust, high-throughput screening of anti-cancer compounds. Biotechnology journal 9, 426–434 (2014).
[26.] Fang, Y. et al. Rapid generation of multiplexed cell cocultures using acoustic droplet ejection followed by aqueous two-phase exclusion patterning. Tissue Engineering Part C: Methods 18, 647– 657 (2012).
[27.] Ashby, W. J. Developing a system of scalable complexity for in vitro models of cell migration. (Vanderbilt University, 2012).
[28.] Muenzner, J. K. et al. Generation and characterization of hepatocellular carcinoma cell lines with enhanced cancer stem cell potential. Journal of cellular and molecular medicine 22, 6238–6248 (2018).
[29.] Avtanski, D. B. et al. Indolo-pyrido-isoquinolin based alkaloid inhibits growth, invasion and migration of breast cancer cells via activation of p53-miR34a axis. Molecular oncology 10, 1118–1132 (2016).
[30.] Jensen, S. S., Petterson, S. A., Halle, B., Aaberg-Jessen, C. & Kristensen, B. W. Effects of the lysosomal destabilizing drug siramesine on glioblastoma in vitro and in vivo. BMC cancer 17, 178 (2017).
[31.] Pansa, M. F. et al. Contribution of resident and recruited macrophages to the photodynamic intervention of colorectal tumor microenvironment. Tumor Biology 37, 541–552 (2016).
[32.] Mellor, H. R. & Callaghan, R. Accumulation and distribution of doxorubicin in tumour spheroids: the influence of acidity and expression of P-glycoprotein. Cancer chemotherapy and pharmacology 68, 1179–1190 (2011).
[33.] Zheng, C. et al. Semaphorin3F Down-Regulates the Expression of Integrin αvβ3 and Sensitizes Multicellular Tumor Spheroids to Chemotherapy via the Neuropilin-2 Receptor in vitro. Chemotherapy 55, 344–352 (2009).
[34.] Nakatsu, M. N. et al. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1☆. Microvascular research 66, 102–112 (2003).
[35.] Nakatsu, M. N. et al. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1☆. Microvascular research 66, 102–112 (2003).
[36.] Nakatsu, M. N. et al. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1☆. Microvascular research 66, 102–112 (2003).
[37.] Kaji, H., Kawashima, T. & Nishizawa, M. Patterning cellular motility using an electrochemical technique and a geometrically confined environment. Langmuir 22, 10784–10787 (2006).
Related Products
There are currently no products tagged to this resource.