Authors: Claudia Schwartz and Katrin Höck
Lonza Cologne GmbH, Koeln, Germany
While live-cell imaging has been restricted to costly, high-end devices, the CytoSMARTTM Lux offers an affordable and easy-to-use alternative for virtually any lab. The CytoSMARTTM Lux can be set up in minutes, enabling untrained users to quickly perform their own time-lapse recordings. Images and videos can be easily accessed and retrieved from the CytoSMARTTM cloud portal. Advanced functions, such as reporting of cell confluency via a graphical readout, and the option to use automatic email alerts, can be applied to inform the user when certain culture conditions are reached (for example, once the cell culture has the desired confluency). Hence, the CytoSMARTTM Lux can be used in many different ways to facilitate cell culture work and research.
in the text below several examples of applications of the Lux will be shown.
- Culturing cells in hypoxic conditions
- Standardizing cell culturing conditions
- The effect of confluency on transfection efficiency
Hypoxic Culture of Cells with the CytoSMART™ Device As an Easy-to-use Monitoring System
The use of hypoxic (i.e., low oxygen) culture conditions for cell cultures gains more and more interest in research as a means to mimic the in vivo situation more closely. Whereas physiological in vivo oxygen concentrations can range from 1% to13%, most cell cultures are maintained at ambient 21% O2. Many cellular responses to hypoxia depend on hypoxia-inducible factors (HIFs), which are stabilized under low oxygen conditions and bind the hypoxia response elements (HREs) in the genome, acting as transcription factors, inducing a wide variety of genes. [1]
For example, the HIF pathway has been shown to enhance vascular endothelial growth factor (VEGF) expression in cancer tissue to promote angiogenesis. Furthermore, it plays a major role in obesity-related type 2 diabetes, and has been demonstrated to initiate the metabolic switch from aerobic respiration to anaerobic glycolysis, a mechanism that is also taking place during the reprogramming of somatic cells into iPSCs.[1]
Figure 1. Image of human mesenchymal stem cells (PT-2501) cultured under normoxia taken with the CytoSMART™ Lux 10X System.
Therefore, hypoxic culture conditions are increasingly applied in stem cell culture and cancer research as well as in toxicological and metabolic studies.
Hypoxic Culture in Cancer Research
Hypoxia frequently occurs in the center of solid tumors where the presence and formation of blood vessels is often abnormal or limited. Hence, low oxygen conditions play a key role in regulating cancer cell metabolism and the deeper understanding of this correlation may advance our understanding of cancer formation and the development of potential new therapies. Studies that fail to take account of the actual physiological levels of oxygen in tissues (approximately 5%) and tumors (approximately 1%) may fail to identify the real circumstances driving tumor response to treatment and/or malignant progression. This can be of particular importance in genetic in vitro studies when comparison to human tumor tissue is required.[2]
For example, the treatment of colorectal cancer cell lines with the cancer drug AW464 under normoxic vs. hypoxic culture conditions showed that hypoxia sensitizes colorectal cells to the drug. Furthermore, a comparison to normal cells, such as endothelial cells or fibroblasts (both from Lonza), revealed drug potency against endothelial cells but not fibroblasts, suggesting possible anti-angiogenic activity of the drug.[3]
Hypoxic Culture in Stem Cell Research
Hypoxic culture conditions are thought to enhance the generation of iPSCs, at least partially, as HIFs could be shown to increase pluripotency-related gene expression.[4] Recently, Mathieu et al. were able to show that the metabolic switch from aerobic respiration to anaerobic glycolysis takes place early during reprogramming. HIFs are necessary to initiate this metabolic switch, and the stabilization of HIFs during early phases of reprogramming is sufficient to induce the switch to By Claudia Schwartz and Katrin Höck, Lonza Cologne GmbH, Koeln, Germany Live Cell Monitoring: Examples of the Effects of Environment Control and Confluency on Cell Cultures While live cell imaging has been restricted to costly, high-end devices, the CytoSMART™ Lux offers an affordable and easy-touse alternative for virtually any lab. The CytoSMART™ Lux can be set up in minutes, enabling untrained users to quickly perform their own time-lapse recordings. Images and videos can be easily accessed and retrieved from the CytoSMART™ cloud portal. Advanced functions, such as reporting of cell confluency via a graphical readout, and the option to use automatic email alerts, can be applied to inform the user when certain culture conditions are reached (for example, once the cell culture has reached the desired confluency). Hence, the CytoSMART™ Lux can be used in many different ways to facilitate cell culture work and research. in the text below several examples of applications of the Lux will be shown. • Culturing cells in hypoxic conditions • Standardizing cell culturing conditions • The effect of confluency on transfection efficiency Figure 1. Image of human mesenchymal stem cells (PT-2501) cultured under normoxia taken with the CytoSMART™ Lux 10X System. Appnote 0001 glycolytic metabolism.[5]
Culture and expansion of mesenchymal stem cells (MSCs) is typically performed under ambient O2 concentration, regardless of the metabolic milieu of the niche in which they normally reside. Since scientists have started to study the impact of hypoxia on MSCs, the positive effect on self-renewal and differentiation potential has been clearly demonstrated. Various signaling pathways are involved in the hypoxia-induced cell functions, but HIFs are the key regulators of the cellular response to hypoxia.[6]
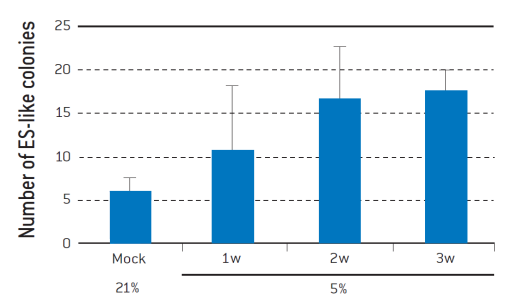
Figure 2. Hypoxia increases the efficiency of iPSC generation from human dermal fibroblasts.
Four reprogramming transcription factors were introduced into adult human dermal fibroblasts. After 6 days, the cells were trypsinized and seeded onto the feeder layer of mitomycin C-treated STO cells. The cells were cultivated under 5% O2 for 7 (1w), 14 (2w), or 21 (3w) days. The number of human ES-like colonies was determined on Day 32 after reprogramming.[4]
Cell Monitoring Under Hypoxia
While hypoxic conditions can be easily established using specialized incubators, the monitoring of cell cultures under hypoxic conditions has been extremely restricted until now. An online-monitoring system that allows direct monitoring of the cell culture in the incubator would be the preferred option. Thus far, however, this option has not been readily available to researchers. The CytoSMART™ Lux overcomes this issue in that it allows continuous online monitoring of cell cultures under hypoxia. With its small size (133 x 90 x 100 mm), the Lux fits into virtually any specialized incubator. What is more, as the cell culture can be viewed via the CytoSMART™ cloud portal, the monitoring of hypoxic cultures becomes completely independent of the presence of the researcher in the lab.
Standardized Cell Culture Can Be Easily Achieved Using the CytoSMART™ System
A standardized cell culture is not easily obtained as biological systems do not always behave reproducibly, e.g., in particular primary cell systems, such as patient-derived materials, may widely vary in proliferation behavior. In addition, every cell biologist will vouch for the fact that cell culture performance is highly user-dependent and requires a certain degree of expertise to achieve consistency in cell cultures. While not always easy to maintain, standardized cell culture is of great importance, as the condition of a cell culture may have a significant influence on the downstream results obtained with the cultured cells.
One way to obtain a more standardized cell culture is to use cells for any downstream assay at consistent confluency. However, the determination of cell confluency is very user-dependent. The CytoSMART™ Lux overcomes this issue in offering a user-independent analysis of the cell confluency which is recorded in the CytoSMART™ Project Page (here referred to as cell coverage). In addition, automatic alerts can be set so that the researcher receives an email notification once the cell culture has reached a chosen confluency, offering a very simple yet effective tool to easily standardize cell culture.
Effects of Different Cell Culture Confluencies on Transfection Efficiency
The confluency of a cell culture can have a great effect on transfection efficiencies. In the example below, neonatal human epidermal keratinocytes were transfected using Lonza’s Nucleofector™ Technology at 40% and 70% confluency (see Figure 3). As can be seen, the transfection efficiency with pmaxGFP™ shows a 20% difference in GFP expression.
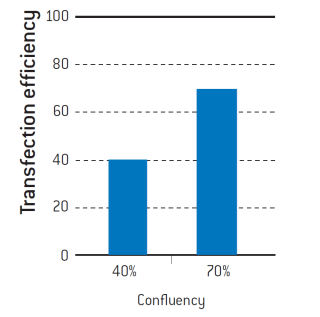
Figure 3. Neonatal human epidermal keratinocytes (Lonza, 192906) were cultured until they reached 40% and 70% confluency. Cells were transfected with pmaxGFP™ using the Nucleofector™ Technology under the same conditions. Transfection efficiencies were determined by flow cytometry.
This variation in transfection efficiency may affect the results of any downstream assay significantly, e.g., with higher standard deviations of the assay and, in consequence, a possible need for more repeated experiments to obtain an assay result with acceptable CV.
Figure 4. Images of neonatal human epidermal keratinocytes at 40% and 70% confluency taken with the CytoSMART™ Lux 10X System.
Summary
With the new CytoSMART™ System, live cell monitoring has become easy and affordable. Documentation and recording of a cell culture can be performed for a multitude of purposes, and at any given time and place, by using the CytoSMART™ Connect Cloud Service in conjunction with internet access.
References
[1] De Miguel et al. (2105) Curr Mol Med 15(4):343–59.
[2] McKeown (2014) Br J Radiol 87, 1035.
[3] Mukherjee et al. (2005) BJC 92, 350–358.
[4] Yoshida et al. (2009) Cell Stem Cell 5, 237–241.
[5] Mathieu et al. (2014) Cell Stem Cell 14, 592–605.
[6] Haque et al. (2013) SciWJ 2013, 632972.
